For example a carbon-oxygen bond is more polar than an oxygen-fluorine bond because the difference in electronegativity for oxygen and carbon is greater than the difference between fluorine and oxygen. The covalent bond is the chemical bond between atoms where electrons are shared forming a molecule.
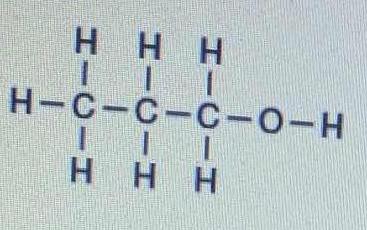
The Bond Between Which Two Atoms Has The Greatest Degree Of Polarity In The Following Molecule Socratic
8 What bond holds atoms together.
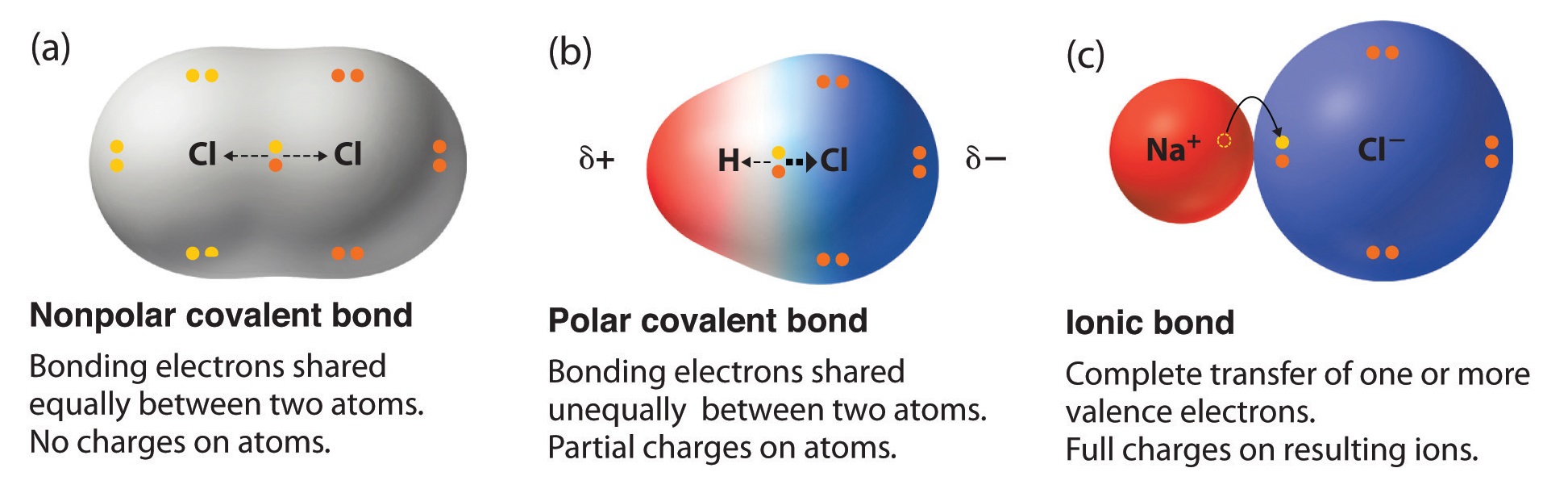
. 12 Why does breaking bonds absorb energy. The quick answer - right from the get-go since nitrogen is one of the most electronegative elements in the periodic table the bond it forms with hydrogen will be the most polar out of all those listed. Identify each bond as either polar or nonpolar.
The bond between which two atoms is most polar 1C-O 2OFF 3он-0. The most common form is the single bond. Those elements that have a greater capacity to attract electrons will be the most electronegative.
The bond between which two atoms has the greatest degree of polarity. Which two atoms in the periodic table form the most polar bond. The O-H bond has the highest electronegativity difference hence it is he most polar bond.
Equally and the resulting bond is nonpolar. The covalent bond is the chemical bond between atoms where electrons are shared forming a molecule. 304 - 22 084.
10 Is bond breaking positive or negative. When electrons are shared between two atoms but there is an uneven of sharing of electrons then the bond between the two atoms is considered. A carboncarbon bond is a covalent bond between two carbon atoms.
Whereas the bond between a metal and a nonmetal is usually ionic. List three examples of pairs of atoms with ionic bonds. The molecule with the polar bond that has the greatest difference in electronegativity is the most polar.
9 How the bonds are formed. Which statement explains why a C-O bond is more polar than a F-O bond. The bond between which two atoms is most polar.
Correct answer to the question The bond between which two atoms is most polar. Questions in other subjects. Carbon atoms can also form double bonds in compounds called alkenes or triple bonds in compounds called alkynes.
The bond between which two atoms is most polar H-O. Explain in terms of electronegativity why an H-F bond is expected to be more polar than an H-I bond. Which formula represents a molecule with the most polar bond.
First electronegativity is a chemical property that measures the ability of an atom to attract electrons in a bond. A bond composed of two electrons one from each of the two atoms. If the difference in electronegativity between two bonded atoms is greater than 21 then the bond is considered ionic.
For the N-H bond. The electrons in a bond between two iodine atoms i2 are shared. 11 What does the process of breaking and reforming bonds allow cells to make.
The correct answer is 4 I and F. If the difference in electronegativity for the atoms in a bond is greater than 04 we consider the bond polar. Covalent bonds are established between non-metallic elements such as hydrogen H oxygen O and chlorine Cl.
List at least three examples of pairs of atoms with nonpolar covalent bonds 11. Which kind of bond would form between two hydrogens. 14 What is released when a bond between two.
Pure covalent bonds have zero electronegativity between the bonding atoms. The electrons in a bond between two iodine atoms I2 are shared. The bond between which two atoms is most polar.
1 point O A covalent bond would form because the electron would be shared so both hydrogens have a full stable shell. Other questions on the subject. What is its average speed.
The electrons in a bond between two iodine atoms are shared. 1 C-N 3 S-Cl 2 H-H 4 Si-O. Atrain travels 74 kilometers in 3 hours and then 81 kilometers in 5 hours.
304 - 22 084. The O-H bond has the highest electronegativity difference hence it is he most polar bond. The bond between which two atoms is most polar H-O.
According to the video if the difference in electronegativity between two atoms is less than 05 then the bond is considered. The polarity of a bond is given by the difference in electronegativity between the two atoms that form said bond. 13 When a bond is formed between two atoms the energy of the system will.
The correct answer is 4 I and F. The chemical bond between which two atoms is most polar. The bond between two nonmetals is usually covalent.
Taking into account the definition of electronegativity and polarity of a chemical bond the H-O bond is the bond with the higher polarity. A polar covalent bond has an electronegativity difference between mathbf04 and. For the N-H bond.
A chemical bond that involves the equal sharing of electrons would be an _____ bond. The covalent bond is the chemical bond between atoms where electrons are shared forming a molecule. O An ionic.
The O-H bond has the highest electronegativity difference hence it is he most polar bond. If the difference in electronegativity is less than 04 the bond is essentially nonpolar If there are no polar bonds the molecule is nonpolar. English 09012021 0100 Fourscore and seven years ago our fathers brought forth on this continent a new nation conceived in liberty and dedicated to the proposition that all men are created equal.
Chemistry 22062019 0300 litttyyyu33411.
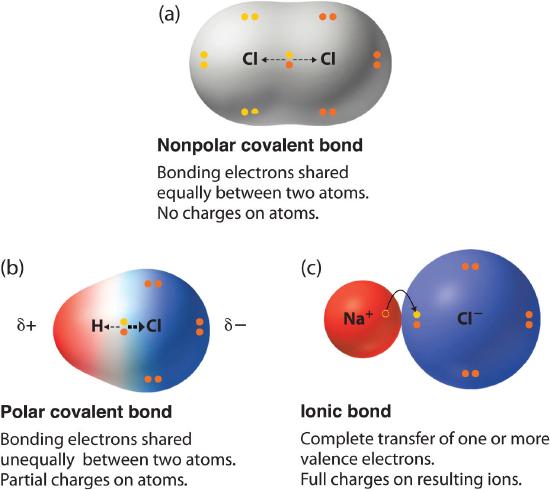
3 6 Electronegativity And Bond Polarity Chemistry Libretexts
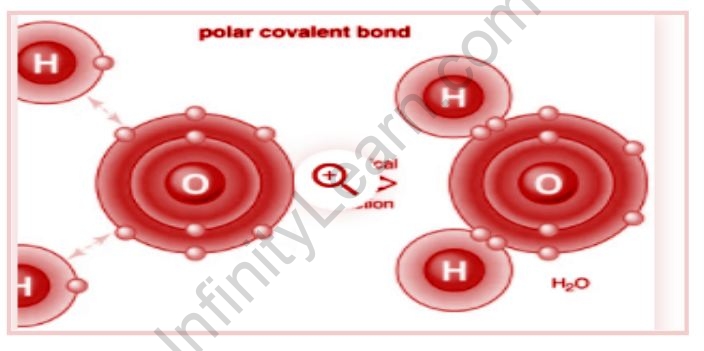
Polar Covalent Bond Infinity Learn

8 7 Bond Polarity And Electronegativity Chi Chemistry Libretexts
0 Comments